Definition
There is no universal definition of isthmocele or standard characterization that clearly indicates its location and size. Most studies refer to isthmocele, cesarean scar defect, niche or diverticulum as a myometrial discontinuity or a hypoechoic triangle in the myometrium of the anterior uterine wall at the site of hysterotomy presented in transvaginal ultrasound (TVUS) or sonohysterography (SHG) examination in non-pregnant women. Also defined as a myometrial thinning, an anechoic defect >1mm or a defect of the myometrium of >2mm at the place of a cesarean scar. The defect usually presents abundant and ectatic vessels, covered in smooth mucosa and menstrual blood often fills the pouch. The site of the defect varies according to the site of the CS, which relates to the stage of labor, uterine cervix changes, and the surgical technique.
Classification
According to the size of the defect: a large defect is described as a myometrial reduction of >50% of the wall thickness or even >80% by some authors. A large defect may also be classified as residual myometrium (RM) <2.2mm by TVUS and <2.5mm by SHG. For management purposes, Marotta et al adopted the cutoff of RM <3mm as a large defect and a RM ≥3mm as a small defect. These radiologic findings can be found incidentally in the absence of symptoms or be associated with clinical symptoms. They can also be classified as asymptomatic or symptomatic when presenting as AUB, pelvic pain, and infertility.
Prevalence
The exact prevalence of isthmocele is unknown and is related to the method used to assess the defect.The prevalence of isthmocele rages from 24% to 70% in TVUS examination and from 56% to 84% in SHG in women with 1 or more previous CS.When compared with asymptomatic patients, the prevalence is higher in those with symptoms.
ETIOPATHOGENESIS AND RISK FACTORS
Several risk factors have been related to the development of the isthmocele. Association between isthmocele and multiple previous CS, retroflexed uterus, and failure to identify all CS scars during repeat CS
A CS conducted in active labor and cervical dilatation >5cm is related to larger isthmoceles.3,4 The association of different uterine sutures and the prevalence of isthmocele is still unclear. Although the single-layer myometrial closure appears to increase the risk of isthmocele development when compared to double-layer closure, it is not significantly associated with larger defects.
The improper closure, or even no closure, of the deeper muscular layer, usually unintentional or related to non-perpendicular sutures and endometrial saving techniques, may lead to an irregular myometrium closure, thus causing the development of isthmocele.
Adhesion development in the hysterotomy scar and the anterior abdominal wall, pulling the edges of the wound and impairing the healing due to those counteracting forces on the uterine scar. This mechanism is even more exuberant in a retroflexed uterus, in which those counteracting forces are increased, potentially decreasing blood flow to the healing tissues.
The presence of an individual/genetic predisposition contributing to an impaired wound healing, poor hemostasis, inflammation or adhesion formation, which may influence isthmocele development.
CLINICAL SYMPTOMS
In general, most isthmoceles are asymptomatic,and found incidentally on ultrasound. Symptoms including abnormal uterine bleeding, postmenstrual spotting, dysmenorrhea, pelvic pain, and infertility have been associated with isthmocele.
Gynecologic Complications
Abnormal uterine bleeding (AUB), mostly characterized as postmenstrual bleeding, is the main symptom related to the presence of an isthmocele, being present in 28.9% to 82% women with isthmocele.
The presence of isthmocele may predispose a deposit of blood and menstrual debris within the defect, associated to reduced contractility of the uterus due to fibrotic tissue around the scar, slowing the drainage of the menstrual flow and causing AUB. Regardless of the source, the presence of blood in the isthmocele is also associated with a higher mucus secretion, which could contribute to postmenstrual AUB.
Postmenstrual spotting is more frequent in patients with larger defects than in patients with smaller defects.
Dysmenorrhea and pelvic pain have also been described in isthmocele. Studies stated a correlation concerning the isthmocele size and pelvic pain and dysmenorrhea. The pain complaints were related to inflammatory infiltration, fibrosis and anatomic disruption of the lower uterine segment.
Therefore, if a patient with previous CS presents any of the symptoms such as above, symptomatic isthmocele should be part of the differential diagnosis and thus investigated.
There is association between isthmocele and secondary infertility The presence of blood in the isthmocele could affect the cervical mucus and sperm quality, obstruct sperm transport and make embryo implantation more difficult, therefore impairing fertility.
Obstetric Complications
The presence of an isthmocele is associated with an increased risk of complications during pregnancy, including placenta previa, accrete/increta/percreta, scar dehiscence, uterine rupture, and cesarean scar ectopic pregnancy. The risk of an isthmocele becoming deficient is related to multiple CS.The overall rate of uterine rupture during a posterior pregnancy does not exceed 2%, however, in larger defects, this risk increases to 5%. It appears that scar thickness in ultrasonographic assessment has no practical use as a prognostic marker of uterine rupture.
One of the rarest complications, the cesarean scar ectopic pregnancy, occurs when the embryo is implanted in the myometrium of the cesarean scar defect. Over the last decades, there has been a rise in the prevalence of the cesarean scar ectopic pregnancy, as well as the CS and therefore isthmocele rates.
DIAGNOSIS
There are no definitive criteria for the diagnostic of isthmocele. Imaging methods including ultrasonography, sonohysterography, hysterography, hysteroscopy, and magnetic resonance imaging can be used.
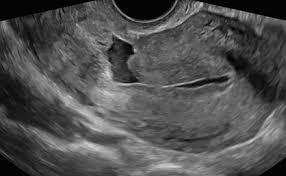
Transvaginal ultrasound (TVUS) is the initial and most usual method described to assess the integrity of the uterus wall in non-pregnant patients. As the principal symptom is postmenstrual bleeding, the early proliferative phase best shows the deposit of blood within the isthmocele, allowing its identification even without the necessity of saline or gel infusion and there is minimal chance of pregnancy. The defect has been described on TVUS as an anechoic triangle defect in the myometrium with the base communicating to the uterine cavity, or a deformity (wedge, shape, concavity or sacculation) on the anterior isthmus.
The prevalence of isthmocele in sonohysterography (SHG), when compared with TVUS, appears to be higher (56%-84% against 24%-70%). Nevertheless, SHG is more sensitive than TVUS and the defect seems larger or deeper by SHG. Therefore, the saline infusion sonohysterography (SIS) is more sensitive and specific for the identification of isthmocele by filling the defect and providing contrast. When compared to the TVUS, SIS presented better results by detecting more defects and more often classifying them as larger on average of 1 to 2mm.
Hysterography (HSG) can also assess the isthmocele; however, it cannot measure the myometrial thickness, which is a limitation of this method. Moreover, if blood or mucus is accumulated in the isthmocele, HSG may not clearly identify the defect.
Using magnetic resonance imaging (MRI) allows determining the RM thickness of the isthmocele on the sagittal T2-weighted views.
Hysteroscopy enables direct visualization and confirmation of the isthmocele.Usually described as a pouch or a discontinuity of the anterior uterine wall,hysteroscopy allows for visualization and potential treatment.
Treatment
The treatment of isthmocele ranges from clinical management with expectant or pharmacological treatment, surgical treatment, and hysterectomy to sparing techniques including hysteroscopic, laparoscopic, laparotomic, or transvaginal procedures limited to the defect site.The decision to treat takes into consideration the size of the defect, presence of symptoms, secondary infertility and plans of pregnancy.
In the case of incidental diagnosis of asymptomatic isthmocele and no plans for future childbearing, clinical observation and no surgical intervention are usually recommended. In symptomatic women with either AUB, pelvic pain, or secondary infertility, the course of treatment depends upon the size of their defect.
Clinical Management
Expectant treatment is an option for women with small isthmoceles (RM≥3mm).
First choice of treatment for symptomatic isthmocele is the resection of the defect hysteroscopically.
Hysteroscopy
Hysteroscopic resection of isthmocele is a minimally invasive, non-time-consuming and low morbidity procedure, allowing visualization and repair of the defect. The surgical technique overall consists of the resection of fibrotic tissue from the defect, presented like a flap underneath the triangular pouch. The resection of the niche edges setting the wall in continuity to the cervical canal improves the flow drainage and prevent the retention of menstrual blood. Fulguration of the base of the pouch, either globally or targeting visible vessels, enables the removal of the inflamed and congested tissue, preventing the in situ production of fluid and blood. Uterine perforation and bladder injuries are the major risks of the hysteroscopic procedure. Therefore, in order to reduce this risk, the resectoscope treatment by hysteroscopy is recommended to be performed if the remaining myometrial thickness is >3mm.
Laparoscopy
A laparoscopic approach has been advocated for large defects (RM <3mm), in the presence of symptoms and desire to maintain fertility. Laparoscopic isthmocele repair consists in the resection of the isthmocele edges, in order to excise the scar tissue, closing the defect in two-layer sutures. Laparoscopy enables a better visualization to identify the defect, allowing repair and thus increasing the myometrial thickness.
The combined use of hysteroscopy and laparoscopy offers many advantages. During the laparoscopy, the bladder can be mobilized to offer superior visualization of the isthmocele and thus minimize the risk of bladder injury. Moreover, the cavity can be assessed for diagnosis and possible immediate surgical treatment of other conditions that can cause pain or infertility, such as chronic pelvic inflammatory disease or endometriosis. The hysteroscopy light source transilluminates the pouch providing guidance in identifying the defect by laparoscopy, and the hysteroscopy can also confirm the laparoscopic repair afterward.
Vaginal Procedure
This technique is described as a dissection of the bladder from the cervix and uterus, opening the vesicovaginal space with the identification of the isthmocele. The defect is excised, and the hysterotomy is closed in two layers. The transvaginal isthmocele repair was found to be cost-effective with shorter operation time and comparably more effective than laparoscopy.This approach, however, demands the surgeon be greatly experienced in vaginal surgery in order to avoid damage to adjacent structures and accurately locate the isthmocele in the limited surgical view.
Hysterectomy
Hysterectomy is the curative management for large symptomatic isthmocele in women who do not wish to conceive anymore.Yet, hysterectomy is a major procedure when compared to other minimally invasive approaches available.
ISTHMOCELE AND PREGNANCY
The assessment of the RM in the lower uterine segment (LUS) by ultrasound can be used to predict the occurrence of cesarean scar dehiscence or rupture in future or ongoing pregnancies.the antenatal evaluation of the LUS can be used, as a complementary data alongside other clinical variables, such as number of previous CSs, time between pregnancies, previous vaginal delivery, maternal age, among others, in the decision of a trial of labor after CS or performing a repeat CS. However, when performed in nonpregnant women who wish futue pregnancies, the RM assessment allows the possibility to identify the defects at higher risk, enabling the possibility of correcting the defect before the next pregnancy.