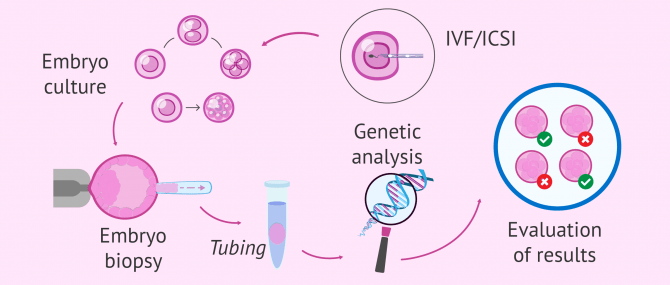
Preimplantation genetic testing is a technique that is used to identify genetic disorders in embryos created through in vitro fertilization or ICSI. Preimplantation genetic screening (PGS) refers to techniques where embryos from presumed chromosomally normal genetic parents are screened for aneuploidy.
PGD and PGS are presently the only options available for avoiding a high risk of having a baby with a genetic disorder before implantation. It is an effective method of preventing heritable genetic disease, thereby eliminating the dilemma of pregnancy termination following unfavorable prenatal diagnosis.
Edwards and Gardner did the first embryo biopsy on rabbit embryos in 1968. In humans, PGD was developed in the United Kingdom in 1980s as an alternative to current prenatal diagnoses. PGD was done for determination of gender as an indirect means of avoiding an X-linked disorder. In 1989 in London, Handyside and colleagues reported the first unaffected child born following PGD performed for an X-linked disorder.
Indications for PGD
Those with a family history of X-linked disorders.
Couples with chromosome translocations.
Carriers of autosomal recessive or autosomal dominant diseases.
Sex-linked disorders
X-linked diseases are passed to the baby through a mother who is a carrier. They are passed by an abnormal X chromosome and manifest in sons, who do not inherit the normal X chromosome from the father. As the X chromosome is transmitted to embryos through the mother, affected fathers have sons who are not affected, but their daughters have a 50% risk of being carriers if the mother is healthy.
Single gene defects
PGD is used to identify single gene defects such as cystic fibrosis, Tay-Sachs disease, sickle cell anemia, and Huntington disease. In such diseases, the abnormality is detectable with molecular techniques using polymerase chain reaction (PCR) amplification of DNA from a single cell. PGD can also be used to identify genetic mutations like BRCA -1, which does not cause a specific disease but increases the risk of a set of diseases.
Chromosomal disorders
The chromosomal disorders includes translocations, inversions, and deletions that can be detected using fluorescent in situ hybridization (FISH). FISH uses telomeric probes specific to the loci site of interest.
Indications for Preimplantation Genetic Screening
Aneuploidy is a major cause for early pregnancy losses . The risk of first and second trimester loss is markedly reduced by transferring healthy embryos.
- Women of advanced maternal age
- Couples with history of recurrent pregnancy loss
- Couples with repeated IVF failure
- Male partner with severe male factor infertility
These patient populations are at risk of failure with IVF because of a high proportion of aneuploid embryos. PGD is thought to decrease this risk by selecting chromosomally normal embryos that have a higher chance of implantation.
Procedure
In order to have embryos to biopsy for PGD/PGS, patients must undergo in vitro fertilization. After fertilization of the egg with sperm, embryos are allowed to develop into cleavage-stage embryos. On day 3 after egg retrieval, a single blastomere is removed from the developing embryo for genetic evaluation of the embryo. Genetic evaluation is performed using PCR, FISH, or comparative genomic hybridization. Nonaffected or normal embryos are then transferred into the uterus for subsequent implantation/pregnancy.
Biopsy procedures
As PGD can be performed on cells from different developmental stages, the biopsy procedures vary accordingly. On unfertilised and fertilised oocytes (for polar bodies), on day three cleavage-stage embryos -for blastomeres and on blastocysts -for trophectoderm cells.
Polar body biopsy.
The sampling of a polar body, which is a small haploid cell that is formed concomitantly as an egg cell during oogenesis, but which generally does not have the ability to be fertilized. Compared to a blastocyst biopsy, a polar body biopsy can be of lower costs and less harmful side-effects.The main advantage of the use of polar bodies in PGD is that they are not necessary for successful fertilisation or normal embryonic development, thus ensuring no harmful effect for the embryo. PB biopsy provides information only about the maternal contribution to the embryo, which is why cases of maternally inherited autosomal dominant and X-linked disorders that are exclusively maternally transmitted can be diagnosed, and autosomal recessive disorders can only partially be diagnosed.
Cleavage-stage biopsy (blastomere biopsy)
Cleavage-stage biopsy is generally performed the morning of day three post-fertilization, when normally developing embryos reach the eight-cell stage. The biopsy is usually performed on embryos with less than 50% of anucleated fragments and at an 8-cell or later stage of development.
The embryo is then anchored on one side with a holding pipette; simultaneously, a small opening within the zona pellucida is made in order to readily access the blastomeres. This opening procedure is called assisted hatching. Assisted hatching can be performed with either a dilute acidic Tyrode solution, with a laser, or with a sharp curette. After the small opening is made, a pipette is placed through the opening and focused on the blastomere of choice, containing a visible nucleus. The blastomere is subsequently gently aspirated into the pipette and expelled into the surrounding medium.
The embryo, now containing one less blastomere, is returned to the incubator into the appropriate culture medium. The blastomere is then processed for either FISH or PCR.
The main advantage of cleavage-stage biopsy over PB analysis is that the genetic input of both parents can be studied. Disadvantage is high rate of mosaicism.
Zona drilling with acid Tyrode’s solution (ZD) was compared to partial zona dissection (PZD) to determine which technique would lead to more successful pregnancies and have less of an effect on the embryo. ZD uses a digestive enzyme like pronase which makes it a chemical drilling method. The chemicals used in ZD may have a damaging effect on the embryo. PZD uses a glass microneedle to cut the zona pellucida which makes it a mechanical dissection method. Studies suggests that using the mechanical method of PZD in blastomere biopsies may be more proficient than using the chemical method of ZD. Currently, zona drilling using a laser is the predominant method of opening the zona pellucida. Using a laser is an easier technique than using mechanical or chemical means.
To overcome the difficulties related to single-cell techniques, biopsy done on embryos at the blastocyst stage, provides larger amount of starting material. If more than two cells are present in the same sample tube, the main technical problems of single-cell PCR or FISH would be overcome.
Studies show that the removal of some TE cells is not detrimental to the further in vivo development of the embryo.
Blastocyst biopsy
Blastocyst formation begins on day 5 post-egg retrieval and has an inner cell mass and the outer cell mass or trophectoderm. At this stage of development, the embryo is formed of more than 100 cells. Human blastocyst-stage biopsy for PGD is performed by making a hole in the ZP on day three of in vitro culture. This allows the developing TE to protrude after blastulation, facilitating the biopsy. On day five post-fertilization, approximately five cells are excised from the TE using a glass needle or laser energy, without loss of inner cell mass. After diagnosis, the embryos can be replaced during the same cycle, or cryopreserved and
transferred in a subsequent cycle.
Drawbacks-
Approximately only half of the preimplantation embryos reach the blastocyst stage. This can restrict the number of blastocysts available for biopsy.
Limited time to perform the genetic diagnosis, making it difficult to redo a second round of PCR or to rehybridize FISH probes before the embryos should be transferred back to the patient.
Tphe potential acquisition of cells from the trophectoderm that are not representative of the developing embryo (inner cell mass) due to mosaicism.
Cumulus cell sampling
Sampling of cumulus cells can be performed in addition to a sampling of polar bodies or cells from the embryo. Because of the molecular interactions between cumulus cells and the oocyte, gene expression profiling of cumulus cells can be performed to estimate oocyte quality.
NON INVASIVE PGD
Studies suggest that transfer of euploid embryos assessed through techniques like comparative genomic hybridization (CGH), next-generation sequencing (NGS) or qPCR based comprehensive chromosomal screening of trophectoderm (TE) biopsies of blastocysts, significantly improves implantation and ongoing pregnancy rates, and decreases miscarriage rates
One of the major limitations of PGS is the presence of chromosomal mosaicism within the developing embryo. Embryonic mosaicism poses significant risk for misdiagnosis as the cells biopsied may not reflect the chromosomal status of the entire embryo. When NGS is used for chromosomal analysis, detection of mosaicism is increased up to 30% compared to aCGH.
Trophectoderm biopsy is technically challenging and invasive .TE biopsy samples typically contain about 4 to 6 cells. Increasing the number of biopsied cells might improve accuracy, but is likely to reduce implantation rate .Non-invasive preimplantation genetic screening (NIPGS) offers an alternative non-invasive approach which avoids embryo biopsy .
DNA has been found in human embryonic blastocoel fluid (BF) and so BF may represent a source of embryonic genetic material that is routinely discarded during the vitrification process to prevent ice crystal formation and improve embryo survival following cryopreservation Compared to TE biopsy, BF aspiration using an ICSI pipette is a less invasive approach to obtain embryonic genetic material, less technically challenging for an embryologist trained to perform ICSI, and more cost effective . A few studies have shown that BF DNA represents to as good an extent, the blastocyst chromosomal composition, when compared with biopsied TE cells and has the potential to be used for preimplantation aneuploidy screening
Cell-free embryonic DNA has also been found in blastocyst culture conditioned medium (BCCM).